Overview
Maximising the Chance of Achieving Diagnoses on Bovine Abortions and a Diagnostic Algorithm Based on Western Canadian Bovine Diseases
Reproductive loss is one of the major problems in cow-calf operations in Western Canada. Although achieving the diagnosis of abortion is undoubtedly important, diagnostic rates had traditionally been low. This article offers some insight to help veterinarians maximize the chance of achieving the diagnosis of abortion based on diseases that are most common in Western Canada. A diagnostic algorithm is also presented aiding veterinarians to understand how a diagnosis is established and how to choose proper diagnostic tests.
1. Collect a thorough list of tissues
Providing sufficient diagnostic materials is key for successful diagnostic investigation. The most important tissue to collect is placenta and every effort should be made to collect it. Also, in abortion storms, one fetus may not be representative. At least two or three abortions should be examined. If it is uncertain whether the abortion is part of the background abortion rate of ~2%, the first one or two can be frozen if snowbanks are available or weather is still cold enough to do so.
Necropsy examination and tissue collection in fetuses is quite standard and a well-trained technologist can perform this task with the supervision of a veterinarian or pathologist. The Bovine Fetus submission form on the PDS Inc. website (https://pdsinc.ca/services/forms.aspx) provides good tissue collection guidance, veterinarians are strongly recommended to consult this resource. Although it looks exhaustive, with exercise, the procedure can be completed within 30 minutes. It is understandable that not all tissues and procedures can be achieved. For examples, sometimes the placenta is not expelled; or it is consumed by the cow or other animals; or some tissues are scavenged. However, following the procedures as much as possible should increase the probability of arriving at a diagnosis.
2. A good diagnostic plan

There are certain diseases/conditions that PDS diagnoses more commonly based on the time of gestation. Our recommended diagnostic strategies below are based on these findings and can help alert you to the timing of the majority of the conditions in Western Canada. Please keep in mind, however, some diseases can occur in both trimesters such as IBR.
| Common diseases | Recommended diagnostic strategies | |
| Second trimester | IBR; various fetal bacterial septicemia; Neospora | HistopathologyBacterial culture (Abomasal fluid, and one of the followings Lung, liver, or kidney)Pre-authorize 2 diagnostic tests at pathologists’ discretion if needed |
| Third Trimester | Vitamin A and/or E deficiencies; trace mineral deficiencies; various chronic non-viral infection, with possible placentitis | HistopathologyVit A and E analyses + mineral panel #1 (Mg, Mn, Fe, Co, Cu, Zn, Se, Mo [offered in PDS Inc.]) on liverBacterial and fungal culture (lung and abomasal fluid)Pre-authorize 1 diagnostic test at pathologists’ discretion if needed |
Reproductive losses in the first trimester do not usually reach the diagnostic laboratories for various good reasons – fetuses can be reabsorbed or are too small to be recognized on the ground even if aborted.
In the second trimester, IBR, various fetal bacterial septicemia and to a lesser degree Neospora are the diseases most frequently diagnosed in Western Canada. Diagnoses of bovine herpes virus 1 (aka. IBR) and bacterial septicemia are relatively straight forward, because of the presence of obvious lesions and the availability of good adjunct tests. The diagnosis of Neospora abortion can be challenging because the lesions and organisms are usually scattered. The presence of non-suppurative encephalitis can be a hint that Neospora is involved, which can be confirmed by IHC. One needs to understand the presence of such a lesion may be missed on just several sections of brain, and the amount of antigen within inflammatory foci may be too little to be detected by IHC. Thoracic fluid of the fetus can be used as a sample for ELISA to detect antibodies against Neospora and BVDV. However, the sensitivity of this method is also a concern. As a result, a positive reaction can confirm fetal exposure to Neospora or BVDV, but a negative result cannot rule it out.
In the last trimester, Vitamin A and/or E deficiencies are frequent laboratory findings. Whether deficiencies of these two vitamins alone are the causes of abortion can be difficult to verify. Squamous metaplasia of salivary ducts (may be seen in Vit A deficiency) and myocardial and/or skeletal muscle degeneration (may be seen in Vit E deficiency) are not usually present. However, these deficiencies may not have to induce morphological changes in order to trigger abortion. Further, their deficiencies are frequently found in combination with various trace mineral deficiencies and can be an indication of a bigger problem such as inadequate protein and/or energy intake. Thus, in our laboratory, Vit A and/or E deficiencies are usually interpreted as significant findings, regardless of whether they accompany morphological changes.
Subacute to chronic non-viral infections, often with placentitis, associated with fungal infection or, various bacterial and protozoal infections are also frequent diagnoses in the last trimester. They are relatively easy to diagnose, unless the placenta cannot be collected for diagnostic purposes. If the placenta is not available, but these infections are suspected, the lung and abomasal fluid can be a good diagnostic alternative as suggested in the table and diagnostic algorithm. Umbilicus can also be useful to include as fixed and fresh tissue. Unfortunately, these alternatives are not a perfect substitute, as some infections do not invade the amnion or the fetus.
3. A diagnostic algorithm
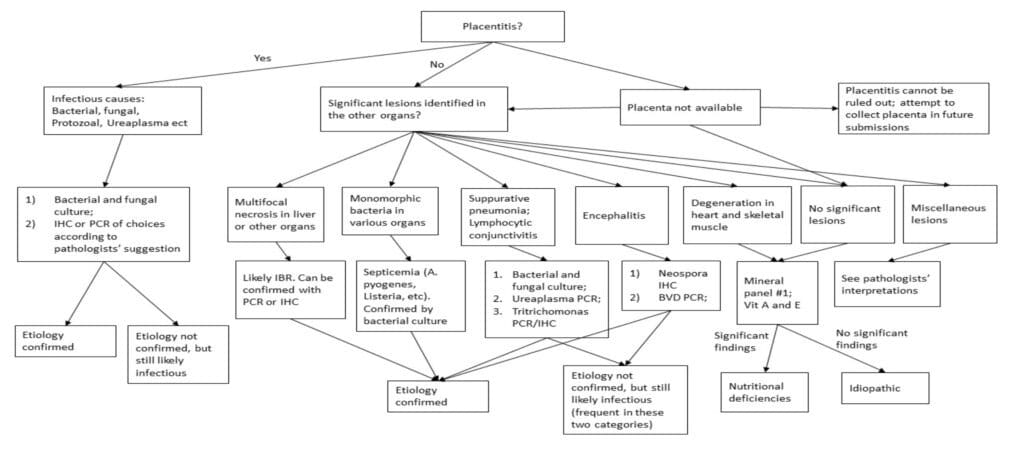
Below is a diagnostic algorithm. Again, this flowchart is designed to cover the more common scenarios and diseases and is not meant to be comprehensive. The algorithm shows the ways different diagnoses are established. With this algorithm, veterinarians can interpret various diagnostic findings and come up with a conclusion. In more complicated cases, veterinarians are recommended to contact the diagnosticians in the laboratory.
4. Submitting samples to PDS Inc.
Complete a PDS submission form. PDS Web Client portal (http://pdsserver.usask.ca/webclient) streamlines completion of the submission form.
- The main “HISTORY” field is the primary location to record vaccination history, treatments, and other notes for herd and individual animal. Gross necropsy notes should be recorded here as well.
- It is important to make sure all fields are filled out. Commodity, Production stage, Primary systems affected and animal age.
- Identifying sample type(s) and quantity on the main page of the submission form, is a required field to create a successful submission. When your sample type is not listed on the suggested samples area you can find all pathological sample types under the dropdown list found with each “sample type “description. “Fresh or Fixed” sample quantities should be listed in this “Samples Sent” field and a description of sample type on the Necropsy form.
- Clearly indicate your testing requests on the submission form and/or pre-authorize adjunct tests.
- To optimize the turnaround time for an entire case, veterinary practitioners can specify on the Necropsy/Histology submission form which, if any, adjunct tests that they would like to have performed; or, alternatively, practitioners can pre-authorize some relevant tests and let the veterinary pathologist decide whether those tests are warranted. Some examples for pre-authorization are: “PCR for IBR, if needed”, “mineral panel #1, Vitamin A and E, if suitable” and “bacterial culture, if indicated”. This would reduce the number of telephone calls made by veterinary pathologists to veterinary practitioner seeking permission to proceed with adjunct testing. The response to these requests may take several days and further delay the timely completion of the report.
- If submitting fixed tissue as well as fresh when only ordering Bacteriology testing, clearly indicate if fixed tissue is to be submitted for pathology or be held for possible future testing. Note: a $20.00 hold fee will apply to hold tissues that do not have pathology work ordered.
- Select the Necropsy/Histology form (found on the bottom righthand corner of the main submission form) to open a second page to use to identify type of fresh tissues being submitted. This is also the best location to direct your requests for adjunct lab test(s).
- After you have “SUBMITTED” the form on-line, print the finalized form with the barcode and include with samples.
Alternative to the WebClient form is to complete the Bovine Fetus submission form found here.
5. Correctly package the fixed and fresh tissues
The basic principle: All tissues for histologic examination should be placed in one or two, formalin-filled, submission jar(s) and each of the fresh tissues should be placed in separate, clearly labeled leakproof bags.

It is counterproductive to place tissues for histologic examination in many separate submission containers. Veterinary pathologists are trained to histologically identify the submitted tissues. When the tissues submitted for histologic examination are placed in separate containers, each of these will be opened at PDS and the tissues pooled into a single container for routine processing.
The opposite applies to the fresh tissues. Place each tissue in a separate, clearly labeled bag to avoid cross contamination and facilitate future identification with adjunct testing.
In winter, freezing artifact can severely hamper the histologic examination of the formalin-fixed tissues. In cold weather, we recommend fixing the tissue in 10% formalin until the tissue is completely fixed (24-48 hrs or longer depending on the size of the tissue) and then transferring the formalin-fixed tissue to 70% isopropyl alcohol (rubbing alcohol) for transport to prevent freezing artifact.
6. Submitting Whole Fetus to PDS
Guidelines for how to package whole fetus submissions to ensure that couriers will deliver your samples to PDS.
- Double bag fetus in heavy duty garbage bags with enough absorbent material in the second bag to absorb any leaks. Ensure the bags are tied closed or closed somehow so they will not leak during shipping.
- Place fetus in a rigid shipping container that will support the weight of the fetus you are shipping. Place absorbent material at the bottom of the shipping container to absorb any potential leaks.
- Suggestions for absorbent material: pee pads and fabric towels work great; paper towel is acceptable; newspaper does not work that well.
- Suggestions for rigid containers: The container must be sturdy enough so that is will not crack from the weight of the fetus or leak during shipping. Heavy duty Rubbermaid containers or heavy-duty plastic garbage containers work well. Styrofoam containers are not great by themselves, a cardboard box around the Styrofoam container helps but we still receive Styrofoam containers that have cracks or are broken even with a box covering it. Unfortunately, we are not able to return the containers for biosecurity reasons.
- Leaking containers are considered a biohazard and couriers may refuse delivery of your samples.
With the information provided in this article, PDS Inc. hopes diagnostic plans for bovine abortions can be easier and clearer for veterinarians.
If you have any questions, contact PDS Inc. at (306) 966-7316.
Calving season is coming, let us be prepared for it!!
