Overview
Prairie Diagnostic Services (PDS) provides detailed submission protocols to ensure accurate, safe, and efficient processing of all diagnostic samples. This section outlines the required information, labeling standards, packaging procedures, and shipping guidelines for submitting biological specimens and whole bodies. Following these protocols helps prevent delays, ensures biosafety compliance, and supports reliable test results. Please review the appropriate submission and packaging instructions before sending samples to PDS.
Submission Form/Sample History
Submission Forms
All submission forms can be found here.
- Submission forms can be generated through the PDS Client Portal (Web Client), or printable forms are available on our Submissions Form page here. Web Client provides access to all the tests offered by PDS, while printable forms contain an abbreviated test selection.
- If using a saved submission form template, ensure all fields are updated with accurate information and test requests before submitting.
- For exotic and wildlife submissions, use the Companion and Exotic Submission Form.
- For submissions involving multiple animals, complete the Multiple Animal Excel Submission Form or provide an Excel spreadsheet listing animal IDs. Ensure all sample IDs are unique and exactly match the sample labels and the spreadsheet. Email the completed Multiple Animal Submission Form or Excel spreadsheet to the PDS Diagnostic Services Office at dso@usask.ca. Ensure samples are organized in the same order as the appear on the animal ID list. Additional charges may apply if samples are not arranged accordingly.
Required Information
Ensure the following information is included:
- Client information: submitting clinic’s name and address,
- Owner’s FIRST AND LAST NAME,
- Premise ID for farm animals,
- Collection date and collection time,
- Animal Identification: Name, all assigned tag numbers (e.g., CCIA/farm); unique markings, tattoos,
- Age (specify days, weeks, months or years, birthdate), species, breed,
- Reason for submission: Diagnostic, Research, Routine Monitoring, Surveillance,
- Sample(s) submitted (ensure sample type matches test requested),
- History,
- Production Stage and Primary Systems Affected for National Surveillance purposes on Bovine, Swine, Equine, Caprine/Ovine, and Avian submission forms,
- Veterinarian name
- Identify test(s) required. Clearly indicate if any samples are to be held for further/future testing (hold fees may apply).
- Include animal’s current vaccination history.
Ensure writing is legible on hand-written forms.
Suspect Risk Group 3 Submissions: When infection with Risk Group 3 organisms is suspected, the form must clearly indicate the suspected organism and any relevant clinical signs. The Suspect RG3 box must be checked on the submission form.
Incomplete Submission: Missing information or paperwork may result in delays and additional charges.
Shipping Paperwork
- Place the submission form in a sealed bag and attach it to the exterior of the secondary package – refer to Sample Packaging Protocol.
- Diagnostic Services Office personnel must be able to access paperwork and sample IDs without direct contact with the specimens. This is an Occupational Health and Safety concern; externally contaminated samples cannot be safely processed.
Externally contaminated samples or submission forms may result in a delay in processing.
Sample Labeling
All samples must be properly labeled with the patient’s identification, the owner’s last name, and source (site). This includes cytology smears and tubes. For cytology smears taken from multiple sites, each slide must be labeled to indicate the corresponding site.
Labeling Blood/Cytology Smears
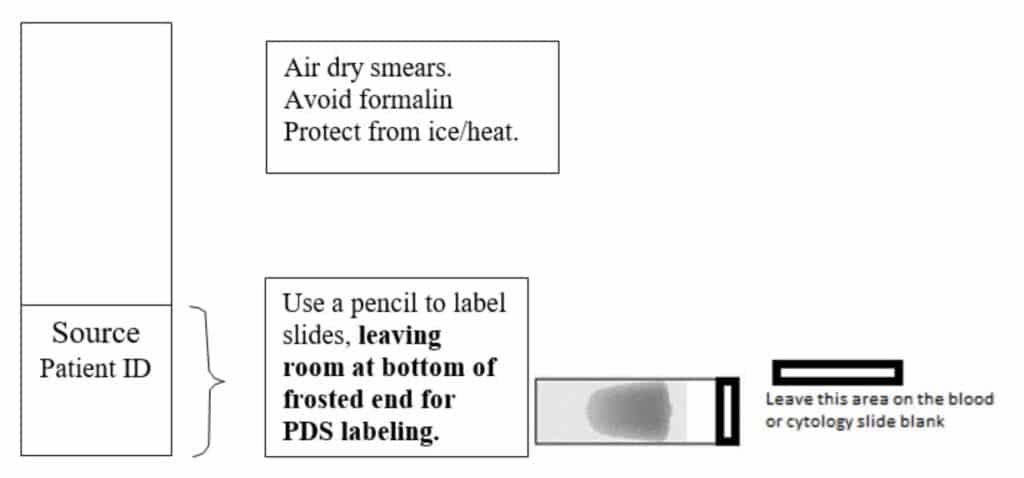
Labeling Sample Tubes
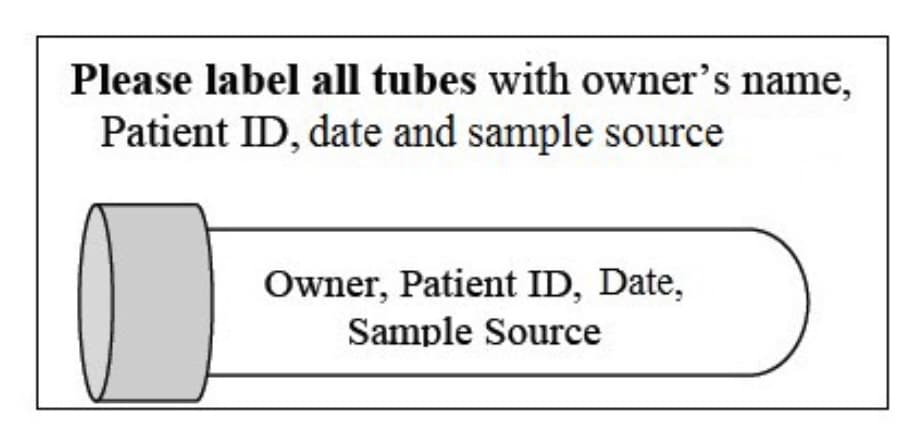
Do not wrap labels around the entirety of the tube, make sure blood level is visible.
Submission Guidelines for Biological Samples
Blood, urine, fluids, feces, swabs, slides, biopsies, etc.
Ensure:
- Each case must include a completed PDS submission form. Clearly label all samples with the owner’s last name, animal ID, sample type (e.g., serum, urine, eye swab, ear swab, feces, plasma, fresh tissue type, fixed tissue type, cytology fluid including fluid site, etc.), and the collection date for EDTA, urine, and cytology fluids.
- If submitting samples from multiple animals, attach a completed Multiple Animal Submission Form or an Excel spreadsheet listing the identifying numbers or names. Email the submission form or spreadsheet to dso@usask.ca. Ensure that all samples are labeled exactly as they appear on the Multiple Animal Submission Form or Excel spreadsheet. When packaging, sort samples in the same order as indicated on the animal ID list. Additional charges may apply for sorting.
- Ensure sample containers are clean and free from contamination with blood or feces. Additional charges may apply if samples are grossly contaminated.
- Submit separate swabs when requesting more than one test that requires the same sample type (e.g., for both Bacteriology and PCR tests on a nasal swab, submit two swabs rather than one). Sharing swabs significantly decreases the chance of isolating target organisms..
- Note: PDS no longer accepting swabs in gel media for PCR testing.
- When submitting urine for complete urinalysis and culture, provide two separate urine samples in sterile containers.
- Use leak-proof, rigid containers with tight-fitting lids, and place these in zip-lock bags – refer to Sample Packaging Protocol below.
- Ensure samples are easily distinguishable from packing materials. Use bubble wrap or syringe containers to protect tubes from breakage, but do not tape samples to bubble wrap.
- Place the submission form in a separate plastic bag to protect it from spills or contamination. If the form is attached to the outside of the box, ensure that couriers do not cover it with shipping labels or information.
- Blood/cytology slides must be clearly labeled – refer to Labeling Blood/Cytology Smears above. Slides must be kept separate from formalin-containing vessels, as contact will adversely affect staining. Package appropriately to prevent breakage during transit.
- If there is an overnight delay in shipping, prepare blood smears and send along with the whole blood. Do not stain the slides before sending.
- Submit 2 good quality blood smears.
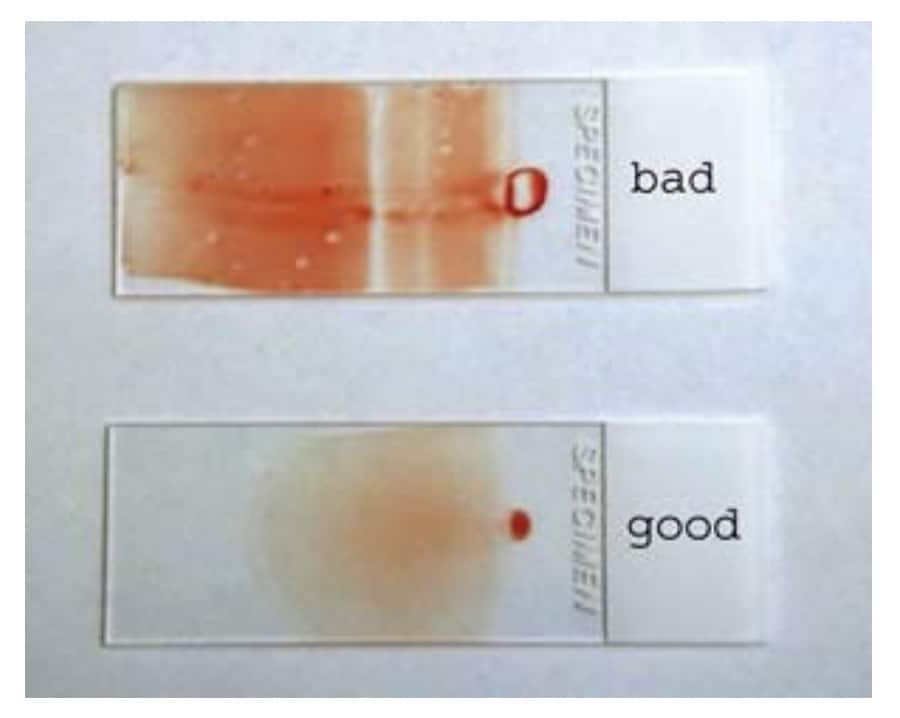
- To prevent freezing during cold weather (temperatures below 0°C), request that the courier protects parcels from freezing. Clearly label the outside of the packaging to indicate this.
Do Not Submit:
- Fecal samples in gloves or milk in whirl-pak bags.
- Syringes with needles or stoppers attached.
- Slides taped to the sides of boxes, as they may be inadvertently discarded.
- Pooled samples from multiple animals in the same bag or jar.
- Sticky labels or tape on glass slides.
Note: PDS has a limited supply of plastic slide holders. Please contact PDS to request holders (shipping charges will apply). PDS no longer provides cardboard slide holders or Styrofoam tube mailers due to biosafety concerns.
Whole Bodies or Portions
Ensure
- A completed PDS submission form accompanies each case.
- Place submission form in a sealed plastic bag.
- For whole bodies, use a large bag followed by a secondary bag with absorbent material.
- For tissue portions, place samples in a leak-proof container (preferably with a screw top for formalin-fixed tissues). Ensure container is labelled with tissue type and contains only one type of tissue.

- Decontaminate the outer bag if necessary.
- Place the sample in a rigid leak proof container (e.g., box, plastic tub). Do not use Styrofoam containers.
- Note: These containers will NOT be returned.
- Do NOT put “body” or “whole animal” on waybill. Purolator will refuse to pick up.
Suspect Risk Group 3 (RG3) Submissions
To safely ship Suspect Risk Group 3 (RG3) samples, which pose serious health risks, please follow these steps:
- Indicate “Suspect RG3” on the submission form.
- Securely package samples to prevent leakage (e.g., double bagging).
- Include the submission form in an envelope or plastic bag. Attach form to the outside of the package.
- Notify PDS prior to sample arrival by emailing dso@usask.ca or calling (306) 966-7316.
RG3 Resources
- Pathogen Safety Data Sheets for organisms (https://www.canada.ca/en/public-health/services/laboratory- biosafety-biosecurity/risk-groups-risk-assessment.html).
- ePATHogen – the Public Health Agency of Canada’s biological agent search tool and a link
- For those working with animal pathogens, visit Canadian Food Inspection Agency’s Disease Agent Information page.
Sample Packaging Protocol
Transport Canada regulations require 3 levels of packaging as follows:
- Primary Container (holds the sample)
- Must be leak proof, plastic, and have a wide mouth opening.
- The exterior of the container must be clean to prevent contamination of the sample.
- Note: A single plastic bag is insufficient for solid specimens over 2 lbs.
- Do not submit liquid samples or solid samples in fluid in plastic bags.
- Secondary Container
- Must be leak proof and contain enough absorbent material to absorb all liquids/specimens in the primary container in case of leakage.
- Must have a wide mouth opening.
- Clean the external surface; decontaminate if necessary.
- Tertiary Packaging
- A rigid cardboard box is acceptable if a sample weighs less than 10 lbs.
- Use a rigid plastic (e.g., cooler), if the specimen weighs over 10 lbs. or contains large volumes of liquid. These containers will NOT be returned.
